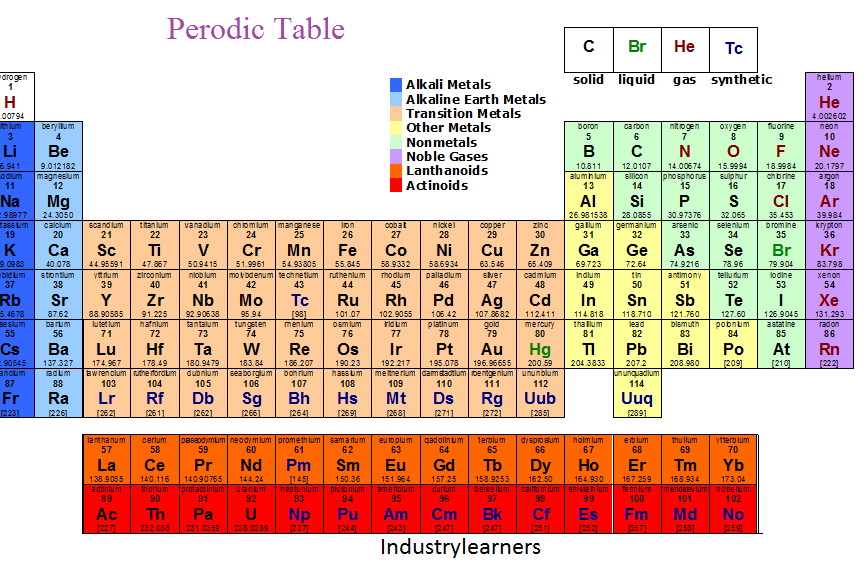
Periodic Table
The Periodic Table
The periodic table is arranged into groups and periods. It is also arranged by metals, non-metals, and metalloids.
Groups
Groups are also called families. These are the vertical columns (up and down). Elements in groups have similar properties. They have the same number of electrons in their outer energy level. The group number (e.g. 1A) is the number of valence electrons.
Periods
Horizontal rows are called periods. Elements in the same period have the same number of energy levels. The row number is the number of energy levels.
Transition Elements (Group 3-12)
• Metals
• Found in the middle of the Periodic Table
• Often occur in nature as un combined elements
• Typically form colour compounds
• Include the inner transitions metals
Inner Transition Elements
• Metals
• Located at the bottom of the chart
• Typically exist in combined forms with other elements, they are formed in laboratories.
• These elements are radioactive